Flames and Kinetics
A comprehensively validated compact mechanism for dimethyl ether oxidation - An experimental and computational study
Addition of oxygenates to diesel fuel has been found to reduce soot emissions and increase its resistance to extinction, which means that a combustion system can be made more stable even at higher strain rates and at the same time operate with reduced emissions. In order to critically evaluate the overall combustion behaviour of DME via numerical simulations, an accurate as well as compact kinetic mechanism consisting of 23 species and 88 elementary reactions is proposed to describe the oxidation of DME in premixed as well as non-premixed systems. This mechanism is further reduced by introducing quasi-steady state assumptions for six intermediate species to finally obtain a 14-step global kinetic scheme. A code is developed in MATLAB to obtain these 14 global steps and their corresponding rate expressions in terms of the elementary reaction rates. (Read More...)
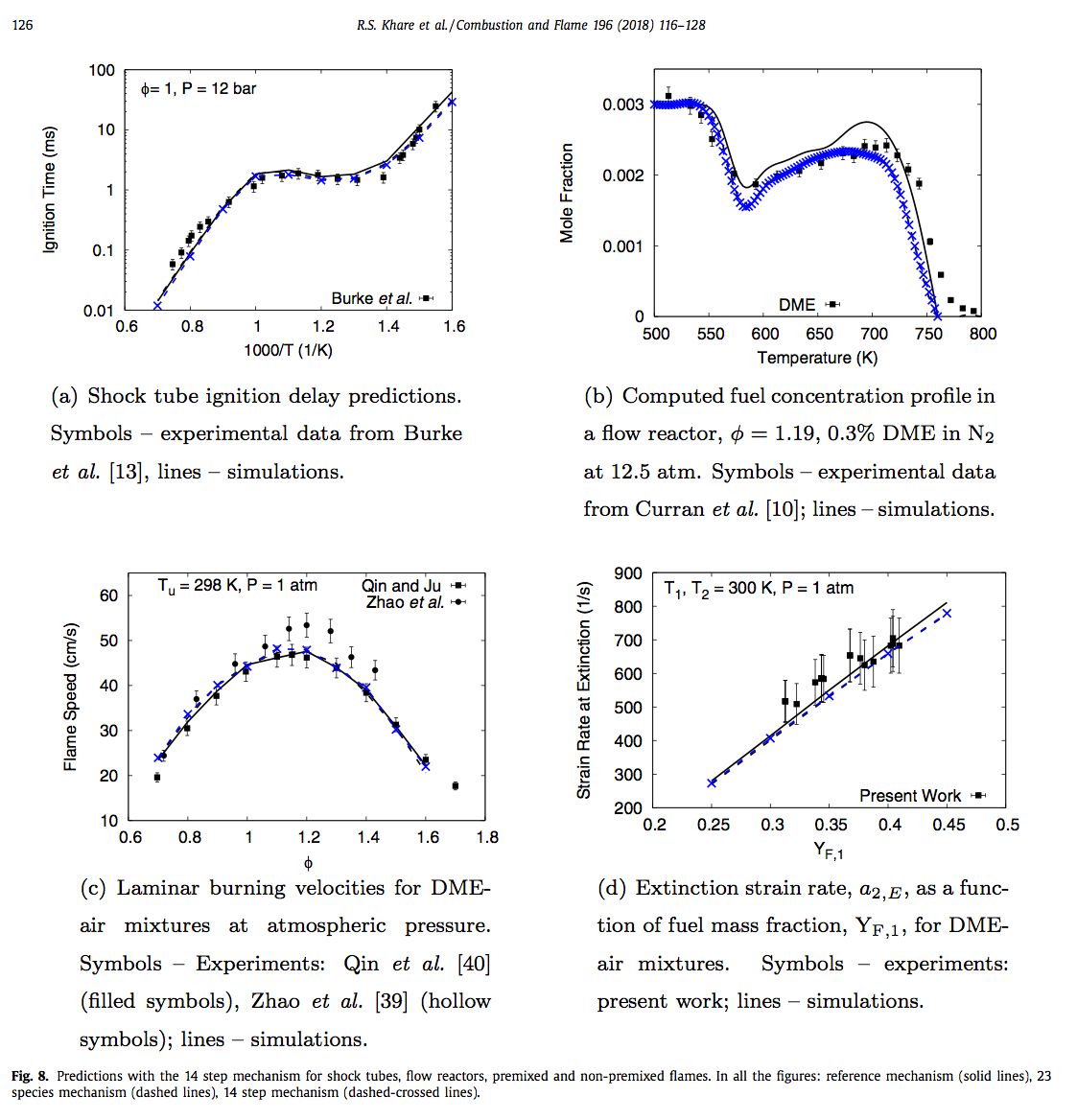
R. S. Khare et al., Combust. Flame, 196 (2018)
Data driven prediction of ignition delays
Ignition delay has an important role in combustion. Ignition delay is affected by different parameters such as temperature, pressure, Equivalence ratio, etc. The objective of the topic is to predict ignition delays of fuels based on the molecular structure and the aforementioned parameters.
Experimental and chemical kinetic modeling investigation of methyl butanoate as a component of biodiesel surrogate
Biodiesel is a potential alternative to fossil diesel. In combustion simulations, in order to circumvent the difficulty in integrating reaction schemes for biodiesels, which are typically of a large size and not well understood, a surrogate approach to simplify the representation of its long chain methylester components is adopted. In this work, a compact reaction scheme for methyl butanoate, which is a potentially important candidate for biodiesel surrogates, is derived, updated with recent literature, and comprehesively validated. The effect of addition of low-temperature chemistry pathways to the methyl butanoate chemical kinetic mechanism has also been explored. (Read More...)
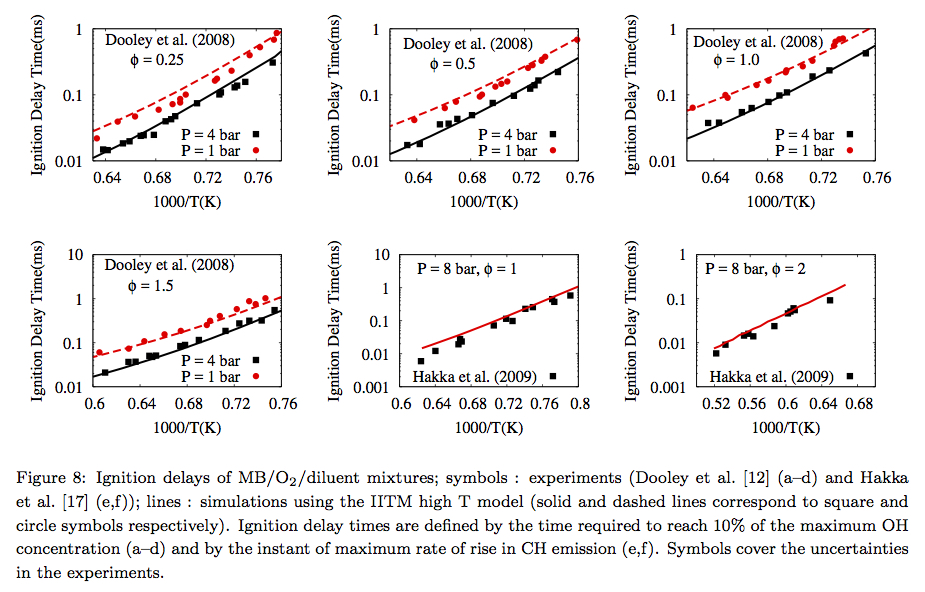
A kinetic modeling based study on the importance of unsaturated ester content in biodiesel surrogates
Biodiesel, a mixture of different methyl esters is one of the potential fuels to replace conventional fossil fuels. There is a need to understand the kinetics of methyl esters. In our recent studies the kinetics of methyl butanoate, a saturated methyl ester has been investigated as it can represent the methyl ester functional group of biodiesel. The composition of biodiesel reveals a significant amount of unsaturated esters and hence unsaturated ester kinetics has to be investigated. In this study, the kinetics of methyl crotonate will be studied leading to the development of a combined biodiesel surrogate formulation comprising of dodecane, methyl butanoate and methyl crotonoate. The importance of unsaturation will be studied and rate rules for larger esters will be formulated. (Read More...)
Compact kinetic model for methylmethacrylate oxidation
Poly methyl methacrylate (PMMA) is widely used in transport and construction sectors. In order to analyze the high temperature gas phase oxidation of PMMA, and thereby predict its fire behaviour with less computational effort, a compact kinetic model for the oxidation of its primary decomposition product, methyl methacrylate (its monomer) CH2=C(CH3)-C(=O)-O-CH3 (MMA), is most essential. As a part of this work, a compact reaction model to describe the oxidation of MMA is developed and validated against fundamental experimental datasets obtained in premixed flames. The final `short MMA mechanism' consists of 88 species and 1092 reactions. (Read More...)
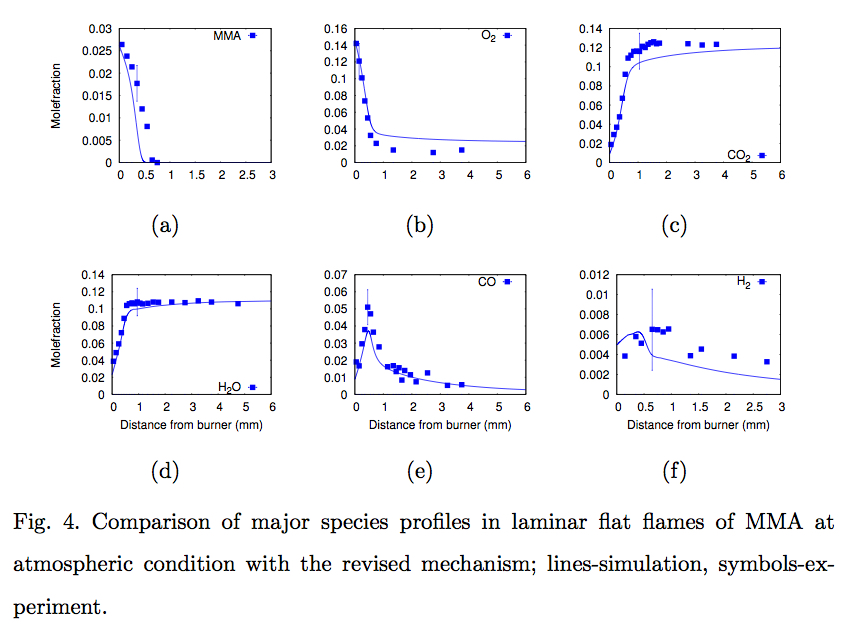
S. Dakshnamurthy et al., Combustion Science and Technology (2018)